"Clinical Trial Supplies Market Report effectiveness of the existing channels of distribution can be uncovered and the best way of distributing the goods to the ultimate consumers can be identified or implemented. This industry report is very useful to all sizes of business which makes it simpler to take informed decisions regarding different facets of the industry. The market insights of this report make the task of planning advertising and sales promotion efforts easy and are also helpful in assessing the effectiveness of advertising programmes.
Global Clinical Trial Supplies Market, By Services (Storage, Manufacturing, Packaging and Labelling and Distribution), Clinical Phase (Phase III, Phase II, Phase IV, Phase I), Therapeutic Uses (Oncology, Cardiovascular Diseases, Dermatology, Metabolic Disorders, Infectious Diseases, Respiratory Diseases, CNS and Mental Disorders, Blood Disorders, Others), End User (Contract Research Organizations, Pharmaceutical and Biotechnology Companies) – Industry Trends and Forecast to 2031.
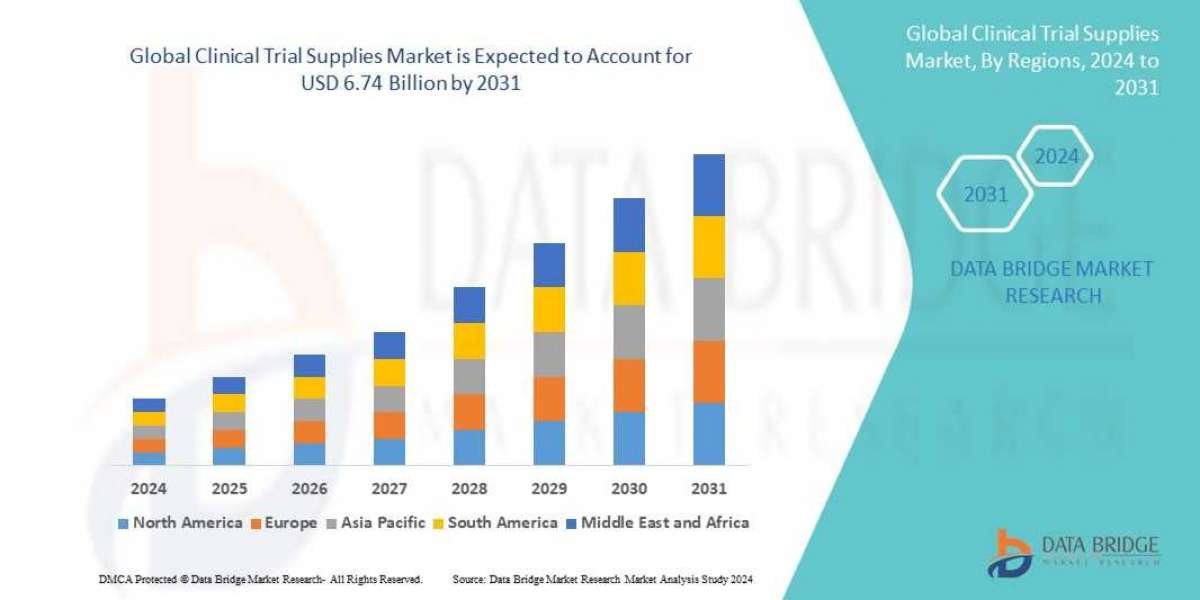
Data Bridge Market Research analyses that the global clinical trial supplies market, which was USD 3.53 billion in 2023, is expected to reach USD 6.74 billion by 2031, and is expected to undergo a CAGR of 8.4% during the forecast period of 2024 to 2031. This indicates that the market value. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Access Full 350 Pages PDF Report @
https://www.databridgemarketresearch.com/reports/global-clinical-trial-supplies-market
Clinical trial supplies refer to the materials and equipment essential for conducting medical research on new treatments or interventions. These supplies encompass medications, placebos, medical devices, and ancillary items required for the trial's execution. Ensuring timely and accurate provision of these resources is critical for the integrity and success of the trial.
Market Players Covered:
Movianto (U.S.), Sharp Services, LLC (U.S.),Thermo Fisher Scientific Inc.,(U.S.), Catalent, Inc (U.S.), PCI Pharma Services (U.S.), Almac Group (U.K.), PAREXEL International Corporation (U.S.), Bionical Emas (U.K.), Alium Medical Limited (U.K.), Myonex (U.K.), Clinigen Limited (U.K.), Ancillare, LP (U.S.), SIRO Clinpharm Private Limited (India), Clinigen Clinical Supplies Management (U.S.), Biocair (U.K.)
Countries Studied:
- North America (Argentina, Brazil, Canada, Chile, Colombia, Mexico, Peru, United States, Rest of Americas)
- Europe (Austria, Belgium, Denmark, Finland, France, Germany, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden, Switzerland, United Kingdom, Rest of Europe)
- Middle-East and Africa (Egypt, Israel, Qatar, Saudi Arabia, South Africa, United Arab Emirates, Rest of MEA)
- Asia-Pacific (Australia, Bangladesh, China, India, Indonesia, Japan, Malaysia, Philippines, Singapore, South Korea, Sri Lanka, Thailand, Taiwan, Rest of Asia-Pacific)
Key Coverage in the Clinical Trial Supplies Market Report:
- Detailed analysis of Clinical Trial Supplies Market by a thorough assessment of the technology, product type, application, and other key segments of the report
- Qualitative and quantitative analysis of the market along with CAGR calculation for the forecast period
- Investigative study of the market dynamics including drivers, opportunities, restraints, and limitations that can influence the market growth
- Comprehensive analysis of the regions of the Clinical Trial Supplies industry and their futuristic growth outlook
- Competitive landscape benchmarking with key coverage of company profiles, product portfolio, and business expansion strategies
TABLE OF CONTENTS
Part 01: Executive Summary
Part 02: Scope of the Report
Part 03: Research Methodology
Part 04: Market Landscape
Part 05: Pipeline Analysis
Part 06: Market Sizing
Part 07: Five Forces Analysis
Part 08: Market Segmentation
Part 09: Customer Landscape
Part 10: Regional Landscape
Part 11: Decision Framework
Part 12: Drivers and Challenges
Part 13: Market Trends
Part 14: Vendor Landscape
Part 15: Vendor Analysis
Part 16: Appendix
Browse Trending Reports:
Buttocks Augmentation Market
Anti Rickettsial Treatment Market
Barbecue Bbq Sauces And Rubs Market
Bottle Display Packaging Market
Transcathetar Devices Market
Citrus Extract Market
Artificial Organ Bank Market
Brown Sequard Syndrome Treatment Market
Bread Maker Market
Concealed Weapon Detection Systems Market
Orthodontic Headgear Market
Bioresorbable Scaffolds Market
Betanin Market
Biotinidase Deficiency Market
Colostrum Market
Citral Market
Facial Tissue Paper Market
Adhesive Foam Tape Market
Pfeiffer Syndrome Market
Acaiberry Extract Market
Brugada Syndrome Treatment Market
Sitosterolemia Market
Disposable Slippers Market
Clinical Communication Software Market
Antimicrobial Nanocoatings Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475