The global cell gene therapy manufacturing services market is expected to reach USD 11.5 billion by 2027, growing at a CAGR of 17.5% from 2022 to 2027. Growing prevalence of cancer and other other diseases, the surge in pharmaceutical RD spending along with partnerships and agreements between pharmaceutical companies and CDMOs are some of the factors driving the market growth. On the contrary, the significant operational costs associated with cell and gene therapy manufacturing is expected to negatively impact the cell gene therapy manufacturing market during the forecast period.
Request for Free Sample Report
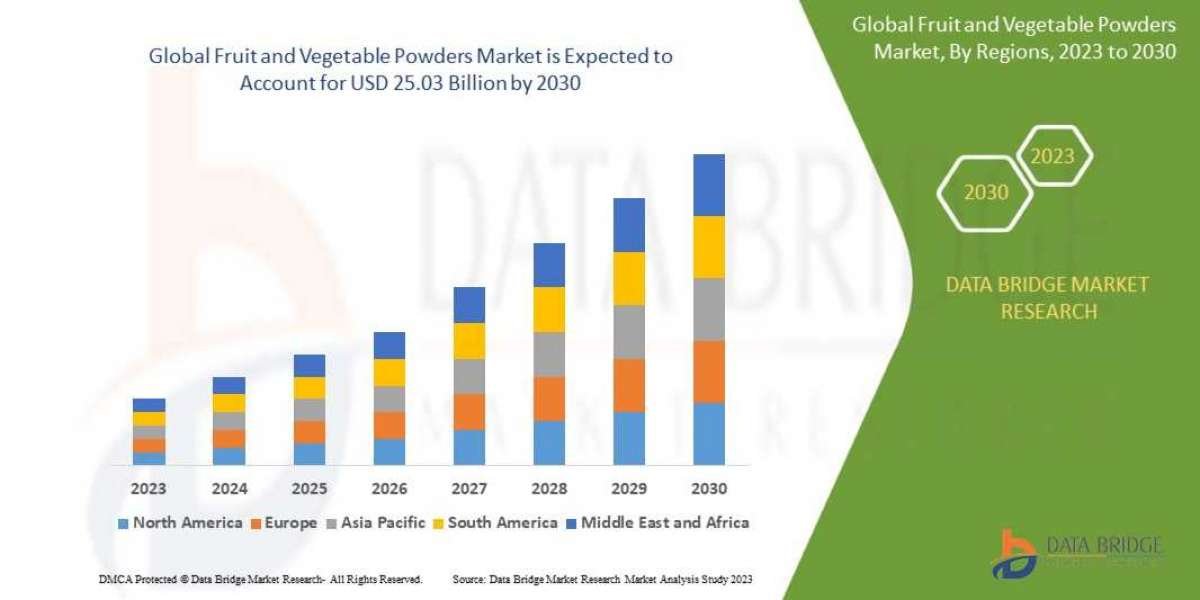
Key Takeaways
- The global cell gene therapy manufacturing services market is expected to reach USD 11.5 billion by 2027, growing at a CAGR of 17.5% from 2022 to 2027.
- The cell therapy segment dominated the cell gene therapy manufacturing services market in 2021.
- North America was the largest regional market for cell gene therapy manufacturing services market in 2021.
- Lonza (Switzerland), Catalent (US), Thermo Fisher Scientific (US), Charles River Laboratories (US), WuXi AppTec (China), Merck KGaA (Germany), Takara Bio Inc. (Japan), Oxford Biomedica (UK), Cell and Gene Therapy Catapult (UK) are some of the key players in the cell gene therapy manufacturing services market.
Market Drivers
- Growing prevalence of cancer and other other diseases
- Surge in pharmaceutical RD spending
- Partnerships and agreements between pharmaceutical companies and CDMOs
- Expansion of cell and gene therapy manufacturing capacities by CDMOs
Market Restraints
- Significant operational costs associated with cell and gene therapy manufacturing
- Stringent regulatory requirements
- Lack of skilled professionals
Market Opportunities
- Increasing demand for cell and gene therapies
- Growing number of clinical trials for cell and gene therapies
- Development of new cell and gene therapy technologies
Market Trends
- Increasing adoption of cell and gene therapy manufacturing services by pharmaceutical and biotechnology companies
- Growing focus on partnerships and collaborations between pharmaceutical companies and CDMOs
- Expansion of cell and gene therapy manufacturing capacities by CDMOs
Geographical Analysis
- North America was the largest regional market for cell gene therapy manufacturing services market in 2021.
- Europe is expected to be the second-largest market for cell gene therapy manufacturing services market during the forecast period.
- Asia Pacific is expected to be the fastest-growing market for cell gene therapy manufacturing services market during the forecast period.
Company Profiles
- Lonza (Switzerland)
- Catalent (US)
- Thermo Fisher Scientific (US)
- Charles River Laboratories (US)
- WuXi AppTec (China)
- Merck KGaA (Germany)
- Takara Bio Inc. (Japan)
- Oxford Biomedica (UK)
- Cell and Gene Therapy Catapult (UK)
- Genezen (US)
- FUJIFILM Holdings Corporation (Japan)
- Nikon Corporation (Japan)
- The Discovery Labs LLC (US)
- RoslinCT (Scotland)
- JRS Pharma (Germany)
- FinVector (Finland)
- ABL, Inc. (US)
- Resilience (US)
- BioCentriq (US)
- Porton Advanced Solutions (China)
- Andelyn Biosciences (US)
- Forge Biologics (US)
- Vibalogics (US)
- Anemocyte Srl (Italy)
- ElevateBio (US)
Recent Developments
- In 2021, Charles River Laboratories acquired a cell gene therapy CDMO—Cognate BioServices—to increase its cell and gene therapy manufacturing capabilities.
- In 2021, Thermo Fisher Scientific acquired Henogen S.A., Novasep's viral vector manufacturing business in Belgium, for approximately USD 859.7 million. This acquisition will increase its presence in the cell gene therapy manufacturing therapy market.
- In 2019, Thermo Fisher Scientific acquired Brammer Bio, a company engaged in viral vector manufacturing for gene and cell therapies, for USD 1.7 billion.